An Atom's Mass Number Is Equal To The Number Of
Click to see full answer. The sum of electrons plus neutrons in the atom the number of protons neutrons and electrons in an atom the sum of the protons plus neutrons in the atom the number of electrons times the mass of an electron.
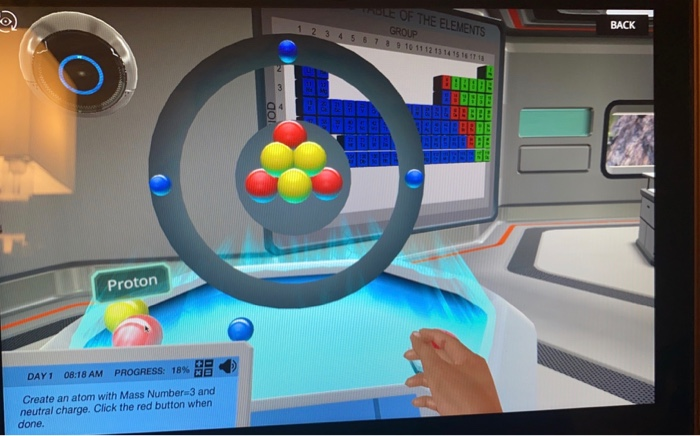
Score 10 120 Day1 08 17 Am Progress 18 Create An Chegg Com
So the mass number of an atom is equal to the number of nucleons.

An atom's mass number is equal to the number of. And the number of particles present in the nucleus is referred as mass number Also called as atomic mass. An atom s mass number equals the number of a. The mass number is equal to the sum of the number of protons and neutrons located within the nucleus of an atom.
Atomic mass the quantity of matter contained in an atom of an element. Who did the Hittites worship. In this scale 1 atomic mass unit amu corresponds to 1660539040 1024 gram.
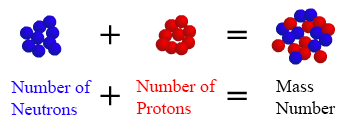
Number of nucleons number of protonnumber of neutron. Mass number is denoted by A. Chemistry The mass number of an atom is equal to the number of protons the number of nucleons.
The number of protons plus neutrons. The atomic number of. The mass number - the atomic number.
Nucleus are subatomic particles located in the nucleus of atoms. A number of protons in the nucleus of the atom B number of electrons and protons in the atom C number of neutrons and protons in the nucleus of an atom D number of neutrons in the nucleus of an atom. Chemistry When Hydrogen starts burning in air it.
What is the mass number of an atom of potassium that has 20 neutrons. The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus. The Correct Answer is.
Answer verified by Toppr. Mass number is the sum of number of proton and number of neutron in the atom. Mass of total positive charge present in an atom is 16533 times to that of mass of electron.
The number of protons and electrons the number of neutrons ANSWER. They are not exact matches on the. Consider a neutral atom with 30 protons and 34 neutrons.
It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom 1992646547 1023 gram which is assigned an atomic mass of 12 units. Protons plus the number of electrons. Z is the atomic number.
A mass number of an atom is equal to the. Protons neutrons Hence the mass number of an atom is equal to the number of protons plus neutrons. The number of neutrons in an atom is equal to.
MASS NUMBER. The number of protons plus the number of neutrons comprise almost all of the mass of the atom because electrons have a negligible mass. Mass number of an atom is equal to total number of nuc.
Find the atomic number of an element. Both proton and neutron are present in the nucleolus pf the atom and are called nucleons. Take note that the nucleus of an atom is composed of protons and neutrons.
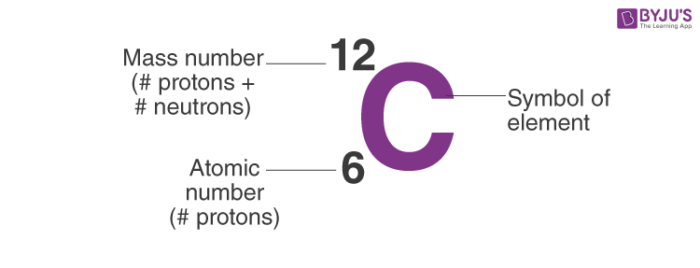
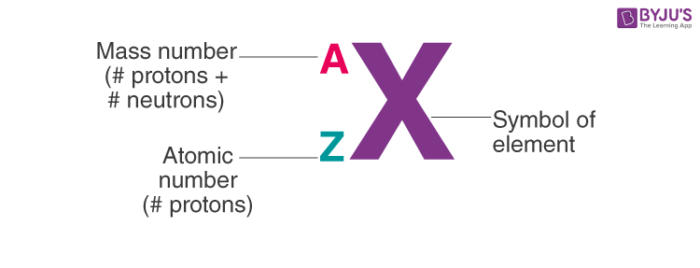
Thereof how can you calculate the number of neutrons N in an atom. A is the mass number. Click hereto get an answer to your question The mass number A of an atom is equal to the.
The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus. Mass number is equal to the number of protons plus the number of neutrons in a nucleus of an atom. The atomic mass is equal to the number of.
In an atom the mass number of an atom is equal to the number of ____. Because a proton and a neutron are both about 1 atomic mass unit 1 amu and the mass number like 235 in U-235 is the total number of protons and neutrons so each atom has mass that is very close to 235 amus. The atomic number of atom is equal to the number of.
The nucleons total number of nucleons in the nucleus of an atom is called the mass number of that atom. An atom is made of three subatomic particles. The mass number of an atom is equal to ______.
The mass number of an atom can be calculated from. Protons plus the number of neutrons.

Pin By Sclee Dever On Chemistry Education Atomic Number Chemistry Education Atom
Nondestructive Evaluation Physics Atomic Elements

My Three Favorite Elements Element 1 Boron B Atomic Number 5 Atomic Mass Numb Physical Science Middle School Matter Science Atom Activities

The Nucleus The Center Of An Atom For Dummies Atom Atomic Number Chemistry

The Atom Chemistry Is My Jam Atom Protons Chemistry Classroom
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs

3 4 Atomic Mass And Atomic Number Chemistry Libretexts Atom Atomic Number Mass Number

One Mole Of A Substance Is Equal To 6 022 10 Units Of That Substance Such As Atoms Molecules Or Ions The Dimensional Analysis Number Videos Molar Mass

The Atom Chemistry Is My Jam Atom Chemistry Help Chemistry Classroom
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs

The Structure Of The Atom Boundless Chemistry

Chalkboard With Description Of Periodic Table Notation For Neon There Is A Square With Three Values In It Chemical Elements Periodic Table Atomic Number Atom
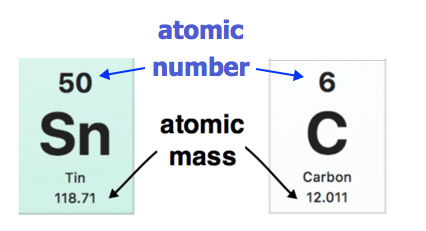
2 6 Atomic Masses Chemistry Libretexts

Notes Isotopes And Ions Warm Up Write In Packet P 6 Writing Packet Mass Number

Atomic Number Atomic Mass And Isotopes Article Khan Academy

Mass Number Definition Overview Expii
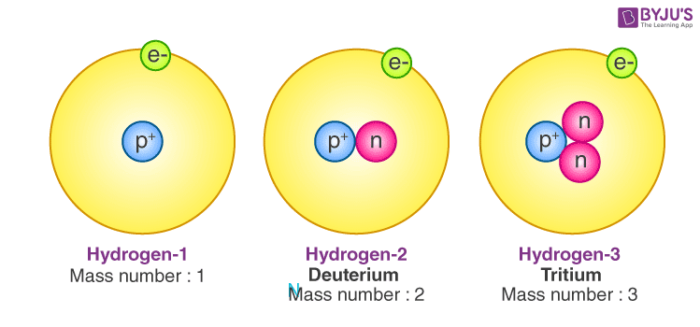
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs

Easy Steps To Find Out How Many Protons Electrons And Neutrons In An Atom Mums Crib Sheet Science Chemistry Homeschool Science Physical Science

Post a Comment for "An Atom's Mass Number Is Equal To The Number Of"