What Is Mass Number Of An Element Equal
The mass number of an element is equal to the number of neutrons added to the number of protons. Its atomic number is 2 so it has two protons in its nucleus.
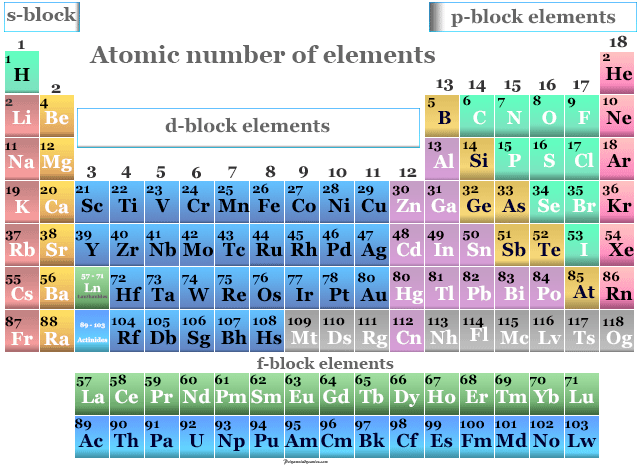
Atomic Number Atomic Mass Elements And Definition
Then add them together.

What is mass number of an element equal. While the mass number is the sum of the protons and neutrons in an atom the atomic number is only the number of protons. A periodic table of elements is needed to complete this task. Its nucleus also contains two neutrons.
The number of neutrons is equal to the difference between the mass number of the atom M and the atomic number Z. The mass number A of an atom is equal to the. The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus.
The mass number is not located on the periodic table. Most of the heavier atoms have a range of isotopes and the quoted atomic mass on the Table is the weighted average of the isotopic masses. Consider the element helium.
The mass number of an isotope is equal to the sum of the protons and neutrons in its nucleus. The atomic mass of an element can be described as. Find the atomic number of the element of which the tubes anticathode is made.
And so for 1H the mass number is simply 1. Lets determine the number of protons neutrons and electrons in the following isotopes. What is the Atomic Mass of Elements.
Elements are defined by the number of protons in the nucleus. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen which consists of a single proton. So A is the number of masses which is equal to the number of protons that is the number of atoms that we symbolized by Z plus the number of neutrons.
For 3H the mass number is simply 3. The mass number in a nucleus is the combined number of protons and neutrons so its protons and neutrons and A symbolizes it. Since 2 2 4 we know that the mass number of the helium atom is 4.
Which is equal to atomic number of an element. The molar mass in grams of a mole for an element is equal to the atomic weight if the element. The atomic mass of an element is equal to the proton number plus the neutron number What is the atomic mass of an element equal to.
Become a member and. The number of neutrons in the atoms of an element is equal to its atomic number. Using the periodic table of elements find the atomic number of the element with mass numbers that need to be calculated.
For 12C the isotopic mass is exactly 12 since the atomic mass unit is defined as 112 of the mass of 12C. Watch learning videos swipe through stories and browse through concepts. Concepts Videos.
To calculate the mass number of a specific element find the number of protons and the number of neutrons that element has. See full answer below. Mass number and isotopic mass The mass number gives an estimate of the isotopic mass measured in atomic mass units u.
Since protons and neutrons account for almost all of the mass of the given atom the atomic mass of a given element is almost equal to its mass number. The isotopes contain 0 1 2 NEUTRONS respectively. The number of neutrons in the atoms of an element is eq.
Elements dont have mass numbers because any given element has isotopes with different mass numbers. The mass number of an element is equal to the number of protons plus the number of neutrons. For other isotopes the isotopic mass is usually within 01 u of the mass number.
The mass number of a nucleus is. For 2H the mass number is simply 2. For example 35Cl 17 protons and 18.

Arrangements In Modern Periodic Table Long Form Of Periodic Table Periodic Table Chemistry Lessons 11th Chemistry

The Atom Chemistry Is My Jam Atom Chemistry Help Chemistry Classroom

The Atom Chemistry Is My Jam Atom Protons Chemistry Classroom

Mass Number Definition Notation Formula Chemistrygod

Balancing Of An Unbalanced Chemical Equation In 5 Easy Steps Chemical Equation Teaching Chemistry Chemical

Section 6 1 Atoms And Moles 1 To Understand The Concept Of Average Mass 2 To Learn How Counting Can Be Done By Weighing 3 Molar Mass Understanding Mole Concept

Average Atomic Mass Easy Science Easy Science Molar Mass Atom

Atomic Mass Question And Solution In 2020 This Or That Questions Relative Atomic Mass Atom

The Nucleus The Center Of An Atom For Dummies Atom Atomic Number Chemistry
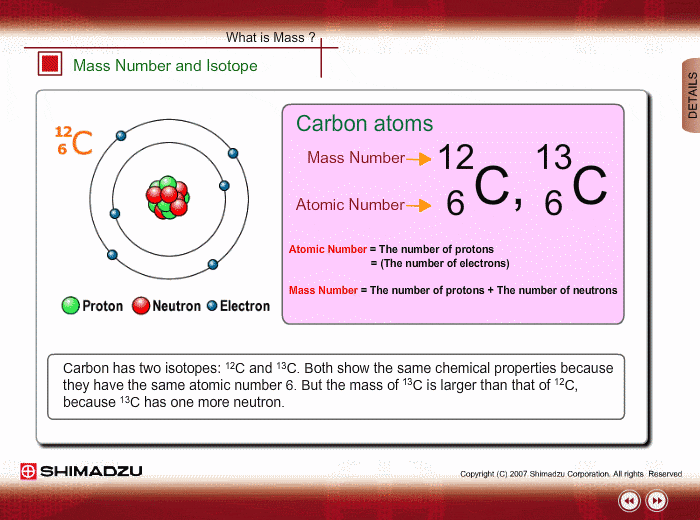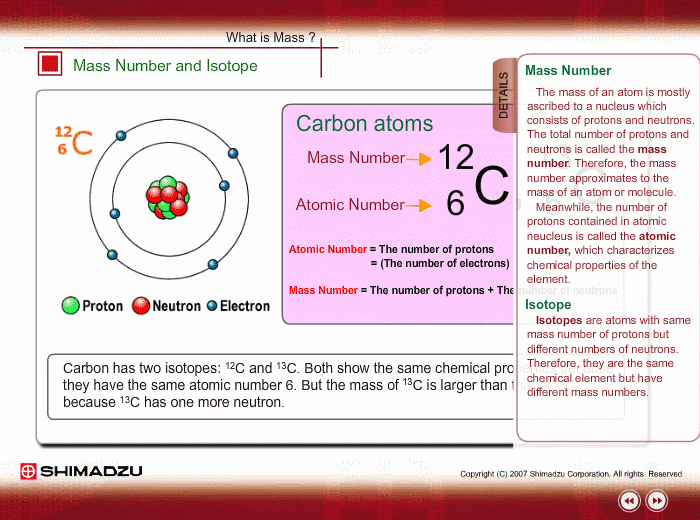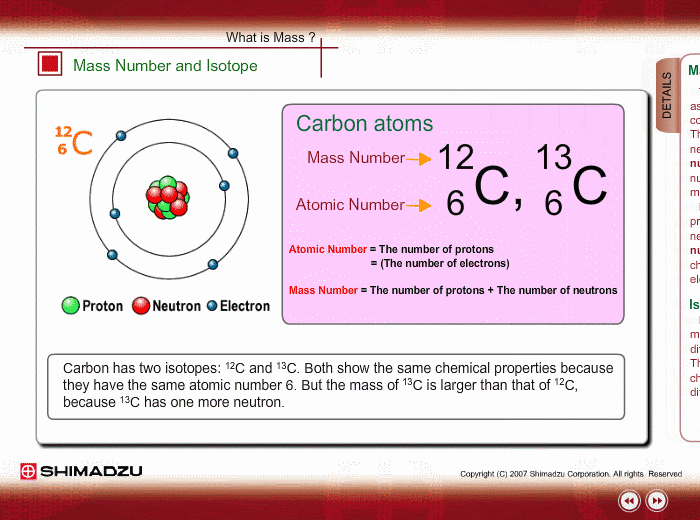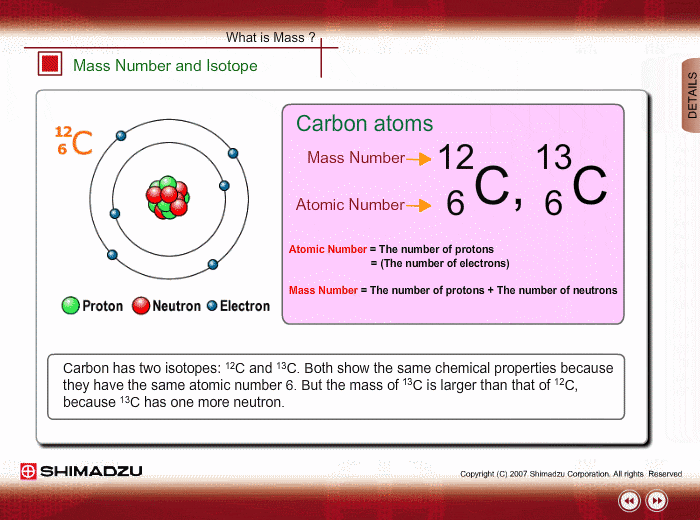
Mass Number And Isotope Shimadzu Shimadzu Corporation

Chem 105 Activity Two Atom And Atomic Structure Physical Science Middle School Montessori Science Atomic Structure

Pin By Makaylah Randolph On Science Chemistry Education Atomic Mass Unit Chemistry

Pin By David Trzcinski On Science Physical Science Periodic Table Earth Science

Pin By Sclee Dever On Chemistry Education Atomic Number Chemistry Education Atom

3 4 Atomic Mass And Atomic Number Chemistry Libretexts Atom Atomic Number Mass Number

Atoms And Molecules Chemistry Lessons Chemistry Classroom Teaching Chemistry

A Section Of The Periodic Table Moving Across Period Two Elements Have The Electronic St Periodic Table Periodic Table Picture Periodic Table Of The Elements

Easy Steps To Find Out How Many Protons Electrons And Neutrons In An Atom Mums Crib Sheet Science Chemistry Homeschool Science Physical Science

Post a Comment for "What Is Mass Number Of An Element Equal"