What Is The Mass Ratio Of Isotopes Of Hydrogen
Tritium 3 H 1 or T The mass ratio of protium deuterium and tritium is 123. Deuterium 2 H 1 or D and 3.

Mass Ratio An Overview Sciencedirect Topics
The ratio is a small whole-number ratio.

What is the mass ratio of isotopes of hydrogen. When H2 is used as the sample gas R R Ki2 where R is the true HDH2 ratio R is the observed mass 3mass 2 ion-current ratio and i2 is the ion current at mass 2. Hydrogen isotopes in water are measured from H 2 gas formed by reducing water with a suitable reducing agent like U Zn Cr or C. Of these 1 H 2 H 16 O and 18 O are abundant and can be easily measured by mass spectrometry.
For a given mass of chlorine compound A contains twice the mass of copper as does compound B. Protium 1 1 H 2. Write the names of the isotopes of hydrogenWhat is the mass ratio of these isotopes.
Deuterium 2 1 H or D and 3. Mass number of two isotopes of an element differ by 2 unit A and A 2. If we decompose an 180 g sample of water we would get 160 g of oxygen 20 g of hydrogen 160 g O 20 H 80 or 801 Ex.
The stable isotopes of hydrogen and oxygen have a long history of use in hydrology and paleoclimatology. Two stable isotopes of hydrogen 1 H and 2 H and three of oxygen 16 O 17 O and 18 O occur naturally in waters and in biological and geological materials. What could be the ratio of the two isotopes.
We measured the isotopic composition of extracted beverage water using both isotope ratio infrared spectroscopy IRIS. Protium 1 H 1 2. Hydrogen has three isotpes.
Protium - H 1 1. Hydrogen has three isotopes in nature. The mass ratio of copper per gram of chlorine in the two compounds is 21.
The mass ratio of protium deuterium and tritium is 1. Tritium 3 1 H or T The mass ratio of protium deuterium and tritium is 123. Hydrogen 2H and oxygen 18O stable isotope analysis is useful when tracing the origin of water in beverages but traditional analytical techniques are limited to pure or extracted waters.
Arnavsingh13 arnavsingh13 Protium deuterium tritium. Specifically wavelength-scanned cavity ring-down spectroscopy and isotope ratio mass. Join Login 11th Chemistry Structure of Atom Isobars and Isotopes Write the names of isotopes.
Tritium mass number 3 often represented by the symbol T is. Number of nucleons in its nucleus. Think about your result.
1H1 1H2 1H3. The isotopes of hydrogen include protium 1 1 H deuterium 1 2 H or D and tritium 1 3 H or T. The atomic mass m r of an isotope nuclide is determined mainly by its mass number ie.
Hydrogen has three isotopes. What is the mass ratio of these isotopes. The H3 factor K is a parameter required in high-precision mass spectrometric analyses of hydrogen isotopic abundances.
It is also possible to exchange hydrogen isotopes between H 2 gas and H 2 O in the presence of Pt catalysts. 140 g N 30g H. Small corrections are due to the binding energy of the nucleus see mass defect the slight difference in mass between proton and neutron and the mass of the electrons associated with the atom the latter because the electronnucleon ratio differs among isotopes.
What is the mass ratio of these isotopes. Summary of isotopes of hydrogen. 2 See answers sarthakrahate24 sarthakrahate24 The mass ratio of protiumdeuteriumtritium is 123.
The most abundant hydrogen isotope has an atomic mass number of 1 but the mass number of 2 called deuterium and often represented by the symbol D is present in small quantities. Ratios of 2 H 1 H to H 2 masses 3 to 2 of the sample hydrogen gas are measured and reported as 2 H values. Write the names of isotopes of hydrogen.
If we decompose a 170 g sample of ammonia a compound composed of nitrogen and hydrogen we would get 140 g of nitrogen and 30 g of hydrogen or a nitrogen-to-hydrogen mass ratio of. Average atomic mass is 05 more than the lower mass number.

Law Of Multiple Proportions Practice Problems Chemistry Examples Fundamental Chemical Laws Youtube

Formula From Atomic Masses Mass Ratio Youtube

A Preliminary Study On Adulteration Control Of Greek Monofloral Honeys Using Isotope Ratio Mass Spectrometry Greek Mass

Mole Ratio Easy Science Easy Science Mole Science Facts
Write The Names Of Isotopes Of Hydrogen What Is The Mass Ratio Of These Isotopes

Write The Names Of Isotopes Of Hydrogen What Is The Mass Ratio Of These Isotopes

2 3 Calculating Atomic Masses Problems Chemistry Libretexts

Mass Ratio Calculation Chemistry For Non Majors

Chemistry Question Calculating The Mass Ratio Of A Chemical Compund Youtube
Http Courses Washington Edu Phys432 Hd Hd Spectrum Pdf

Chemistry Question Calculating The Mass Ratio Of A Chemical Compund Youtube
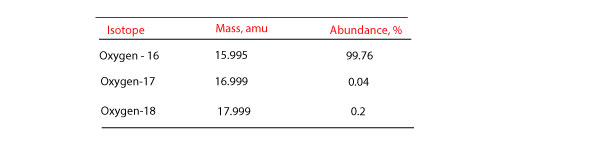
How Do You Calculate Atomic Mass From Percentage Abundance
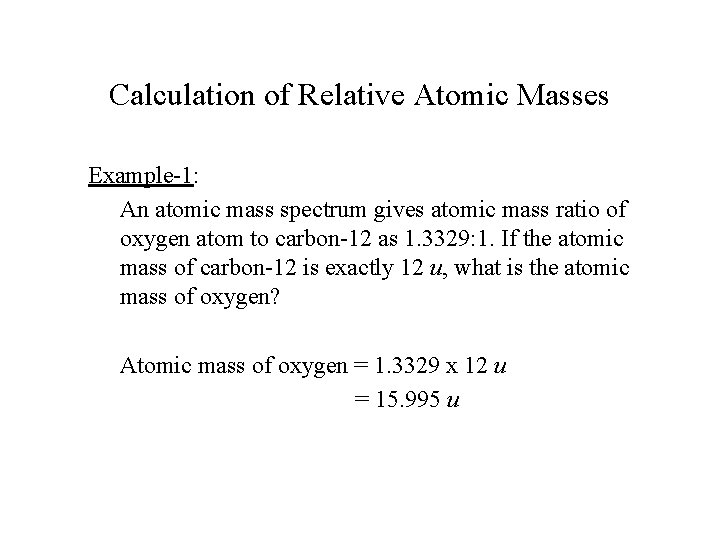
Stoichiometry Atomic Mass The Mole Concept Molar Mass

The Atom Chemistry Is My Jam Atom Chemistry Help Chemistry Classroom

Determining Mole Ratio And Empirical Formula Of Compound Step By Step Template Special Needs Students Worksheet Template Chemistry

Write The Names Of Isotopes Of Hydrogen What Is The Mass Ratio Of These Isotopes

This Week 19th Oct 24th October Is Real Time Chem Week If That Means Nothing To You Check Out Thei Teaching Chemistry Science Chemistry Chemistry Classroom



Post a Comment for "What Is The Mass Ratio Of Isotopes Of Hydrogen"